Abstract
Background: Idiopathic multicentric Castleman disease (iMCD) is an immunologic disorder with unknown etiology that is diagnosed in approximately 1200 individuals in the US annually. Presentation is heterogeneous - ranging from mild/moderate constitutional symptoms to severe multi-organ dysfunction. Treatment guidelines were developed by an international expert panel in 2017 based on the review of a limited number of clinical trials and small case series. Siltuximab, a monoclonal antibody against interleukin-6 (IL6) approved for the treatment of iMCD, is recommended first-line based on evidence from its phase II trial. High-dose steroids and cytotoxic chemotherapy are recommended for patients with severe disease who progress on anti-IL6 therapy. For patients with mild/moderate disease not responding to IL6 blockade, rituximab +/- immunomodulators is recommended. While concurrent steroids are recommended in some cases, steroid monotherapy is discouraged. Limited data exist on the effectiveness of these agents beyond siltuximab. Herein, we evaluate treatment regimens administered to a cohort of 83 iMCD patients with longitudinal treatment and response data.
Methods: A review of cases in the ACCELERATE natural history registry by a panel of experts led to the identification of 83 patients with confirmed iMCD. Longitudinal treatment and response data were abstracted from medical records. Durable treatment response was defined as the best response according to the change in the proportion of abnormal clinical/laboratory criteria sustained for at least 1 year. Severity at the time of treatment initiation was defined as ≥2 of renal failure, fluid accumulation, severe anemia, pulmonary involvement, or hospitalization. Survival analysis for time-to-next-treatment, a proxy for time to treatment failure, was performed on regimens categorized in the guidelines: siltuximab +/- best supportive care (BSC), tocilizumab +/- BSC, rituximab +/- BSC, chemotherapy +/- other agents, and steroid monotherapy. BSC includes steroids, transfusions, and other supportive modalities. Log rank test was used to determine differences across treatment regimens, and pairwise comparisons were Bonferroni corrected.
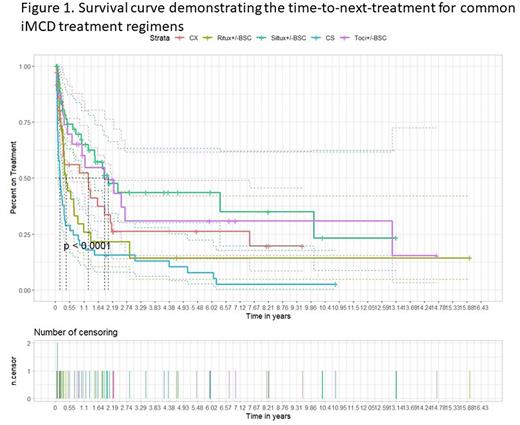
Results: Approximately half (47%) of the patients were female, with a mean (SD) age at diagnosis of 35.9 (16.2). A total of 261 regimens were administered across the cohort. Of the 41 patients who received siltuximab +/-BSC, 44% (N=18) achieved a durable response. Durable response rates to other treatments included: tocilizumab +/-BSC: 41% (7/17); rituximab +/-BSC: 23% (6/26); chemotherapy +/-BSC: 38% (8/21); steroid monotherapy: 6% (2/32). Steroid monotherapy was significantly less likely to induce a durable response than siltuximab +/-BSC (p=0.02). Notably, response rates were significantly lower across modalities in severe compared to mild/moderate disease (p=0.004). We also found that the treatment regimen significantly predicts time-to-next-treatment (p<0.0001, Figure 1). Median time-to-next-treatment for siltuximab +/-BSC is 742 days (95% CI: 1.26, no upper limit), tocilizumab +/-BSC is 689 days (234, no upper limit), rituximab +/- BSC is 153 days (116, 399), chemotherapy +/- other agents is 463 days (107, 791), and steroid monotherapy is 64 days (36,129). Pairwise comparison demonstrated that siltuximab +/-BSC, tocilizumab +/-BSC, and chemotherapy each have a significantly longer time-to-next-treatment compared with steroids (p<0.05). Further, siltuximab +/- BSC also had a significantly longer time-to-next-treatment than rituximab +/-BSC (p=0.02). There were no other differences between regimens.
Discussion: Though guidelines were developed in 2017, this is the first systematic assessment of iMCD treatment regimens included in these guidelines. We found differences in durable response rates and in time-to-next-treatment between regimen approaches. Patients on steroids alone require additional drug-intervention a median of 2 months after treatment start, while patients treated on siltuximab +/-BSC remained on treatment for a median of 2 years before additional interventions were added. Our results support current recommendations to administer siltuximab first-line and limit steroid monotherapy use. These results also demonstrate that additional agents are needed for refractory patients, who have few options and are at risk of death due to progression.
Disclosures
Brandstadter:EUSA Pharma: Consultancy. Lechowicz:ADC Therapeutics: Consultancy; Kyowa Kirin: Consultancy; EUSA Pharma: Consultancy; Secura Bio Inc: Consultancy. Srkalovic:Takeda: Speakers Bureau; Janssen Pharmaceuticals: Speakers Bureau; Foundation Medicine: Speakers Bureau; EUSA Pharma: Speakers Bureau. Lim:EUSA Pharma: Honoraria. van Rhee:EUSA Pharma: Consultancy; GlaxoSmithKline: Consultancy; Karyopharm: Consultancy; Takeda: Consultancy; Janssen Pharmaceuticals: Research Funding; Bristol Myers Squibb: Research Funding. Fajgenbaum:EUSA Pharma: Consultancy, Research Funding.
OffLabel Disclosure:
Our abstract discusses the use of tocilizumab, rituximab, steroids, and cytotoxic chemotherapy for the treatment of idiopathic multicentric Castleman disease (iMCD). These off-label drugs are recommended for patients when siltuximab, the only FDA-approved drug for iMCD, does not induce a response.
Author notes
Asterisk with author names denotes non-ASH members.